A page from the "Causes of Color" exhibit...
Emeralds, alexandrite and self-colored gemstones
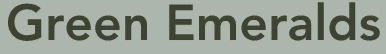
Green emerald. The mineral is transparent emerald, the green variety of beryl on calcite matrix. 2.5 x 2.5 cm. Coscuez, Boyacá, Colombia. Emeralds are the green, gem variety of the mineral beryl (Beryllium aluminum silicate). They are named after the Greek "smaragdos" which means "a light green precious stone," and are generally regarded as among the most precious of gems.
Why are emeralds green?
Here are several postage stamps that have featured the emerald:

Afghanistan |

Colombia |

Colombia
|

Uganda |

Russia |
Emeralds are green for the same reason as rubies are red.
The ruby is a regular crystal of corundum containing chromium as an impurity. The emerald is a regular crystal of beryl that also contains chromium as an impurity. Beryl is the term given to beryllium aluminum silicate (Be2Al2Si6Ol8). In rubies an aluminum atom in the beryl lattice is replaced by a chromium atom. The presence of the chromium impurity makes the colorless corundum crystal red, while the presence of the chromium impurity makes the colorless beryl green. This is because in the emerald crystal, the geometry of the atoms is a little different, as the host molecules interact with the chromium more weakly and the energy differences between the energy levels are reduced, producing green.
The term diagram of Cr3+ in a distorted octahedral field (A), the energy levels and transitions in ruby (B), and the resulting absorption spectrum and fluorescence of ruby (C) and emerald (D). In an emerald, the symmetry is distorted octahedral, but the ligand field is a little weaker: 2.05 eV compared to the 2.23 eV of ruby. This relatively small change produces a significant shift in the absorption bands, as shown at D, thus resulting in a change from the red color of ruby to the green color of emerald. Here, the violet and yellow-red regions of light passing through emerald are absorbed, as in the two upward arrows leading to the 4T1 and 4T2 levels, producing the absorption spectrum shown at D. Green-blue is transmitted to give the color of emerald. Again, the selection rules do not permit a return from the two excited states (4T1 and 4T2) directly to the ground state. In losing its energy again, the Cr3+ must pass through the state labeled 2E with the emission of some heat. In returning from 2E back down to the ground state, a quantum of red light, a red fluorescence similar to that of ruby, is emitted. Interestingly, the position of the 2E level involved in the fluorescence does not change significantly with the ligand field, as can be seen at A, so that the same red fluorescence is present in both red ruby (strongly) and green emerald (weakly).

Red fluorescence. White light (left) and ultraviolet (right) views of a mineral specimen from Franklin, New Jersey, containing calcite CaCO3 (tan color with red fluorescence) and willemite ZnSiO4 (brown with green fluorescence), both containing Mn2+, as well as the green synthetic emerald Be3Si6O18:Cr and the red synthetic ruby Al2O3:Cr, both showing the red Cr3+ fluorescence; the ruby is 2 cm across.
Yellow beryl (Heliodor) is colored by the presence of F3+ ions. Beryl includes emerald, aquamarine, and lesser-known varieties like goshenite (colorless), morganite (pink), Heliodor (yellow), and bixbite (red).
Alexandrite
With such a drastic change in color in going from ruby to emerald resulting from a relatively small change in the ligand field, what might result from half a change in the ligand field? The extremely rare and precious gemstone alexandrite. The absorption bands are so delicately balanced in alexandrite that in blue-rich daylight or the light from a fluorescent-tube lamp we see an intense blue-green color, resembling that of an emerald, while in red-rich candlelight or the light from an incandescent lamp we perceive a deep red color, somewhat like a ruby. Nature has accomplished the nearly impossible task of providing a truly intermediate color between the green of emerald and the red of ruby.

A synthetic alexandrite gemstone, 5 mm across, changing from a reddish color in the light from an incandescent lamp to a greenish color in the light from a fluorescent tube lamp.
The purple-orange dichroism (Cr3+ ligand field colors) in a 3-cm-diameter synthetic ruby; the arrows indicate the electric vectors of the polarizers. One result of the symmetry of the ligand field is "pleochroism," the existence of up to three colors in different directions in a crystal. The two "dichroic" colors, orange and purple, are seen with polarized light in a ruby.
The variation of the ligand field and the color in the mixed system of colorless sapphire Al2O3 and chrome green Cr2O3. As the chromium concentration of a ruby is increased, there is a change from red through gray to green as the ligand field becomes weaker; pure chromium oxide Cr2O3 is the dark-green pigment chrome green. A specimen on the red side of gray can be turned green by heating it to cause a reduction of the ligand field, as the atoms move apart from thermal expansion in "thermochromism"; in the reverse phenomenon "piezochromism," green can be turned red by the application of pressure. Not to scale.
Allotropic and idiochromatic substances
When small amounts of transition metals provide color in otherwise colorless substances, they are termed "allochromatic" (other-colored). Examples include many gemstones, such as the chromium-based ruby, emerald, and alexandrite; the manganese-containing pink-colored morganite form of beryl; and the iron-colored blue and green aquamarines, yellow citrine, and green jade (in part). Some colors in glass, glazes, and enamels are also based on transition-metal compounds or impurities.
"Idiochromatic" substances are those where the color is due to some inherent constituent of the mineral and are referred to as self-colored. They have a limited range of color compared to "allotropic" substances. Idiochromatic and allochromatic transition-metal substances are colored for the same reason, but idiochromatic compounds include pigments such as the green viridian Cr2O(OH)4 and the chrome-oxide green; the blue smalt glass K2CoSi3O8 and Thenard’s blue, Al2CoO4; gems such as pink rhodochrosite MnCO3 and green malachite 2CuCO3.Cu(OH)2; and minerals and ores such as brown manganite MnO(OH), red iron ore Fe2O3, yellow goethite FeO(OH), and green bunsenite NiO.
|
Radiating malachite formations within a host rock |

Reddish brown goethite hosting radiating sprays of olive green laubmannite to 3 cm across. |

Yellow crystals of helvite on quartz and pink rhodochrosite. |