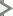A page from the "Causes of Color" exhibit...
Lesson 2 (Gemstones)
Gemstones may be elements, oxides, carbonates, or silicates. Some minerals are too soft to be gemstones, but diamond, corundum, and beryl are not. The exact causes of the green color in emeralds, the blue color in sapphires, the red color in rubies, and the violet color in amethysts are described in detail in this Causes of Color WebExhibit. This lesson is a mixture of online research, hands-on activities, concept mapping, and presenting information to peers. Background information includes:
Learning Outcomes
At the end of these activities, students will be able to:- Make a “nodemap”
- Imagine how to explain topics to other students
- List the main causes of color
- Compare and contrast
- Light made
- Light lost
- Light moved
- Identify the cause of color in gold and gemstones
- Review Moh’s Scale of Mineral Hardness
- Examine the structure of diamonds, rubies, emeralds, sapphires, and amethysts
- Spell new vocabulary words
- Share their node map with friends
Standards and benchmarks
(adapted from NYS High School Standards)
- 1a Minerals have physical properties determined by their chemical composition and crystal structure.
- Minerals can be identified by well-defined physical and chemical properties, such as cleavage, fracture, color, density, hardness, streak, luster, crystal shape, and reaction with acid.
- Chemical composition and physical properties determine how minerals are used by humans.
- 1b Minerals are formed inorganically by the process of crystallization as a result of specific environmental conditions. These include:
- Cooling and solidification of magma.
- Precipitation from water caused by such processes as evaporation, chemical reactions, and temperature changes.
- Rearrangement of atoms in existing minerals subjected to conditions of high temperature and pressure.
- 1c Rocks are usually composed of one or more minerals.
- Rocks are classified by their origin, mineral content, and texture.
- Conditions that existed when a rock formed can be inferred from the rock’s mineral content and texture.
- The properties of rocks determine how they are used and also influence land usage by humans.
S3.1a Organize results, using appropriate graphs, diagrams, data tables, and other models to show relationships.
S3.1b Generate and use scales, create legends, and appropriately label axes.
Presenting science in student-friendly nodemaps
Over the course of the lesson, students will build a nodemap using SpicyNodes, an online tool for making “nodemaps.” These nodemaps are animated, graphic concept maps that present information in small chunks. The final product of this lesson will be a nodemap from individual students or small groups.
A nodemap is a new way to present information. Your students will design a concept map using SpicyNodes, an authoring system that is easy to use. Your students will have their very own URL to access their nodemap. They can email you the URL for you to assess their ability to follow directions, or if you are registered you will automatically see their nodemaps.
Nodemaps are fun and interactive, and are most engaging if they are intended to be shared. Students will learn about color, experiment hands on, and then explain what they learn in a language and style that will appeal to other kids.
Students can include a mixture of text and images in their nodemaps, and organize the nodes in a way that makes sense to them. This exhibit’s node view provides a summary of the entire Causes of Color exhibit using SpicyNodes. In this lesson, students will build their own nodemaps of approximately 30 nodes.
SpicyNodes can be integrated into classroom learning and homework assignments. The open-ended format of SpicyNodes provides a toolkit for learning across disciplines and integrating a variety of subjects. SpicyNodes can be used in all phases of learning - from brainstorming ideas to presenting one’s findings. For example, students can utilize SpicyNodes to break down complex topics into small, manageable, engaging segments. SpicyNodes supports open-ended and nonlinear learning, which is preferred by tech-savvy students. SpicyNodes also encourages a dynamic learning environment by providing virtually unlimited space and scope, as opposed to static and space-constrained concept mapping on paper or Microsoft’s PowerPoint.
Content knowledge for teachers
Light is a form of energy. It originates from many sources, including the sun. Depending on the wavelengths of light, we perceive different colors. Why are there different wavelengths? Why isn’t the world shades of grey? Causes of Color explains all possible causes of color, and how our eye and brain interpret those wavelengths as color. There are four main sections of the exhibit:
- Made - These colors are “made” by new photons of light being created. Topics include: incandescence, fluorescence, fire, lightning, fireworks, vapor lamps, chemiluminescence, bioluminescence, mechanoluminescence, auroras, and Cerenkov radiation. Glowing gases, burning fuels, mixing chemicals, and moving electrons, all produce light of a specific color.
- Lost - These colors are made when some photons are “lost,” and what we see is the remainder. Topics include: water, ice, glaciers, bacteria, metals (like gold), semiconductors, blue diamonds, LED lamps, photoluminescence, red rubies, green emeralds, blue sapphires, amethysts, quartz, chlorophyll, and anthocyanins. Parts of the light are eliminated and you see only a fraction of the whole light wave, that is, a specific color.
- Moved - These colors are made when some wavelengths are “moved” differently than others, causing certain wavelengths to be seen. Topics include: rainbows, dispersive refraction, scattering, interference, iridescence, and holograms. Light waves can be bent, bounced, and reflected, and these phenomena produce different colors.
- Vision - Explanations include: colorblindness, colors for animals (such as birds and horses), early studies of color, representations of color, and melanin. A history of color is delineated and how color is perceived is explained.
The exhibit is a mixture of basic science ideas, which students will be able to immediately understand, and higher-level material that you can explore in depth to give you sufficient background to explain the ideas to your students.
Vocabulary Words
| malleable | ductile | luster |
| metallic bond | covalent bond | delocalized electrons |
| band theory | Fermi surface | electron configuration |
| alloy | anodize | nanoparticles |
| semiconductor | Hope diamond | insulator |
| translucent | conduction band | valence band |
| transition metal | ligand field | corundum |
| beryl | orbital | photon |
| alexandrite | allochromatic | malachite |
| rhodochrosite | goethite | manganite |
| charge transfer | ultramarine | fluorite |
| amethyst | topaz |