About the chemical structure:
| Chemical name: | Zinc(II)-oxide |
| Formula: | ZnO |
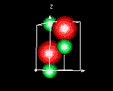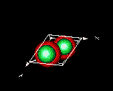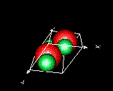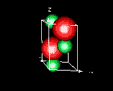
| 3D model: |
red = oxygen, green = zinc |
| Crystal system: | Trigonal - Trapezohedral (at Mineralogy Database) |
| Refractive index: | w = 2.905, e = 3.256; uniaxial |
Color:
| Color Index (C.I.) | PW 4 |
How can you identify Zinc white?
Imaging:
UVF: bright yellow
IRFC: white
OM: Since zinc oxide is derived from smoke fumes, its particles are very fine and are difficult to observe except at very high magnification.

Microscopic appearance at x500 mag
Analytics:
It readily dissolves in alkaline solutions, acids and ammonia without foaming.
Raman spectra: University College London;
FTIR spectra: IRUG
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: very good. Degradation processes: vermilion is, largely, a permanent pigment, its body and hiding power are stronger than those of cadmium red. However in the presence of hydrogen sulphides and sun light the pigment reacts chemically darkening to a black. Although it is a sulphide, it is so inert that it does not darken lead white when they are mixed and as a result they have often been mixed for flesh tints. |
non toxic. Zinc white is not considered to be hazardous, but care should be used in handling the dry powder pigment to avoid inhaling the dust. MSDS: Kremer |
Literature:
Faloon, D. B., Zinc Oxide History, Manufacture and Properties as a Pigment, New York 1925
Artists' Pigments, A Handbook of Their History and Characteristics, Vol. 1: R.L. Feller (Ed.) Oxford University Press 1986, p. 169-186