About the chemical structure:
| Chemical name: | mercuric sulfide |
| Formula: | HgS |
| Crystal system: | Trigonal - Trapezohedral (at Mineralogy Database) |
| Refractive index: | w = 2.905, e = 3.256; uniaxial |
Color:
| Color Index (C.I.) | PR 106 |
How can you identify Vermilion?
Imaging:
UVF: dark blue
IRFC: yellow

Maestro di Marradi, Tuscany, XV-XVI century
The red gown of the Virgin is made of red vermilion which shows up yellow in the IRFC.
OM: Artificial cinnebar does not differ chemically or physically from the natural ore but manufactured vermilion tends to have finer, more uniform particles. Course and broken shard particles are more likely to be natural. Particles are usually between 1-30μm in size.
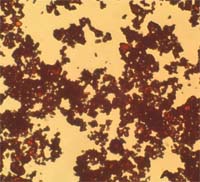
Microscopic appearance at x500 mag
Analytics:
It's identified by means of FTIR, UV/VIS/IR and Raman.
Raman spectra: University College London;
UV/VIS/IR spectra: US Geological Survey
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: very good. Degradation processes: vermilion is, largely, a permanent pigment, its body and hiding power are stronger than those of cadmium red. However in the presence of hydrogen sulphides and sun light the pigment reacts chemically darkening to a black. Although it is a sulphide, it is so inert that it does not darken lead white when they are mixed and as a result they have often been mixed for flesh tints. |
moderately toxic. Some authorities consider natural vermilion, cinnabar, to be non-toxic. Anita Albus writes that the deadly poison of mercury becomes harmless when it is stably bound with sulfur. However, we consider cinnabar and vermilion to be toxic and urge caution in handling the dry powder pigment, as well as the pigment dispersed in medium. MSDS: Natural pigments |
Literature:
Brachert, F. und T., Zinnober, Maltechnik-Restauro, 86, 1980, p.145
Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed) Oxford University Press 1993, p.159-182

